When it comes to advancing research and clinical care for primary mitochondrial diseases (PMDs), the inclusion of patient perspectives is essential.
Mitochondrial diseases present a lot of diagnostic and therapeutic challenges due to the way they can affect patients in so many different ways. Addressing these challenges requires a comprehensive approach that combines not only the top scientific minds in the world but also the lived experiences of patients.
At a recent European Neuromuscular Centre (ENMC) meeting, leading researchers, clinicians and patient advocacy groups gathered to redefine the future of clinical trials and outcome measures for primary mitochondrial myopathies (PMMs), a subset of PMDs whose main symptoms directly involve the muscles. These symptoms may include muscle weakness, tiredness and exercise intolerance.
An outcome measure is simply a way to check if the treatment or intervention being tested is working. It’s what researchers measure to see if there’s a change or improvement.
Shaping the future of clinical trials
The meeting aimed to delve into criteria for diagnosis, explore new digital health technologies and develop patient-centric tools for assessing disease impact and the benefits of new treatments.
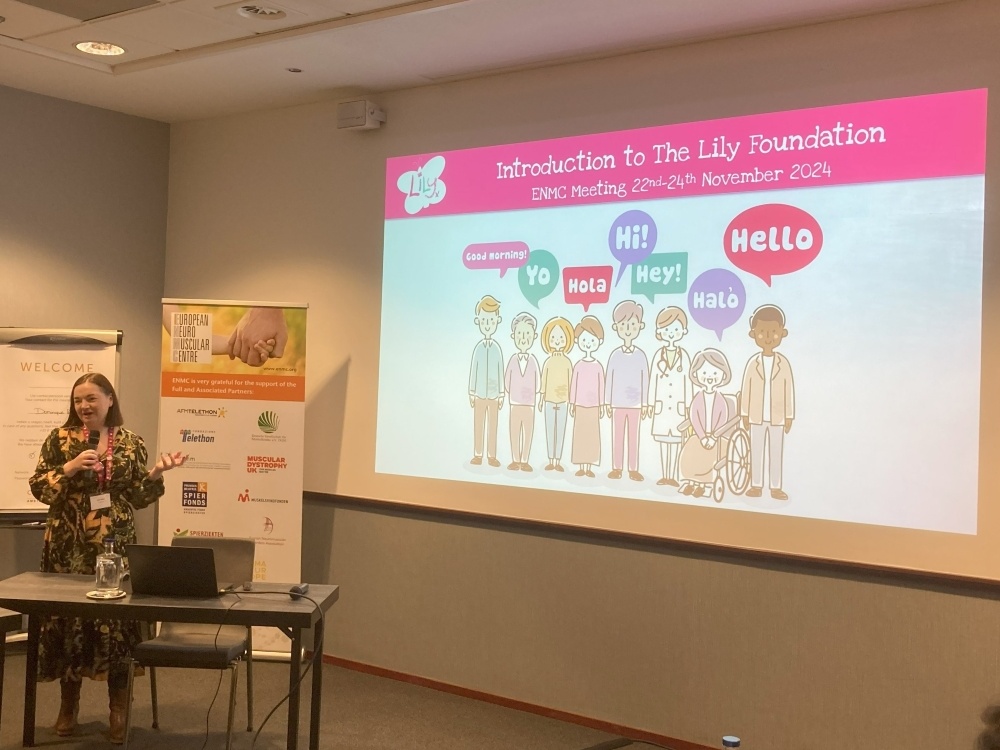
Central to this initiative was the voice of the patient, represented by Katie Waller, Head of Patient Programmes at The Lily Foundation.
Before the meeting, Katie collected the thoughts of 71 patients from not only the UK but across the globe who have a PMM, including those who have taken part in a clinical trial for PMM. This included asking about their lived experiences of participating in a clinical trial, what challenges there may have been and whether the trial was helpful.
Patients were also able to share their thoughts on the future of trials such as leveraging new technologies to track and report symptoms. These patient experiences were to be used as the basis of Katie’s talk to the clinicians at the meeting, ensuring their voices were heard and represented.
The image below shows a snapshot of some of the feedback patients contributed to the survey. You can click on the image to expand it.