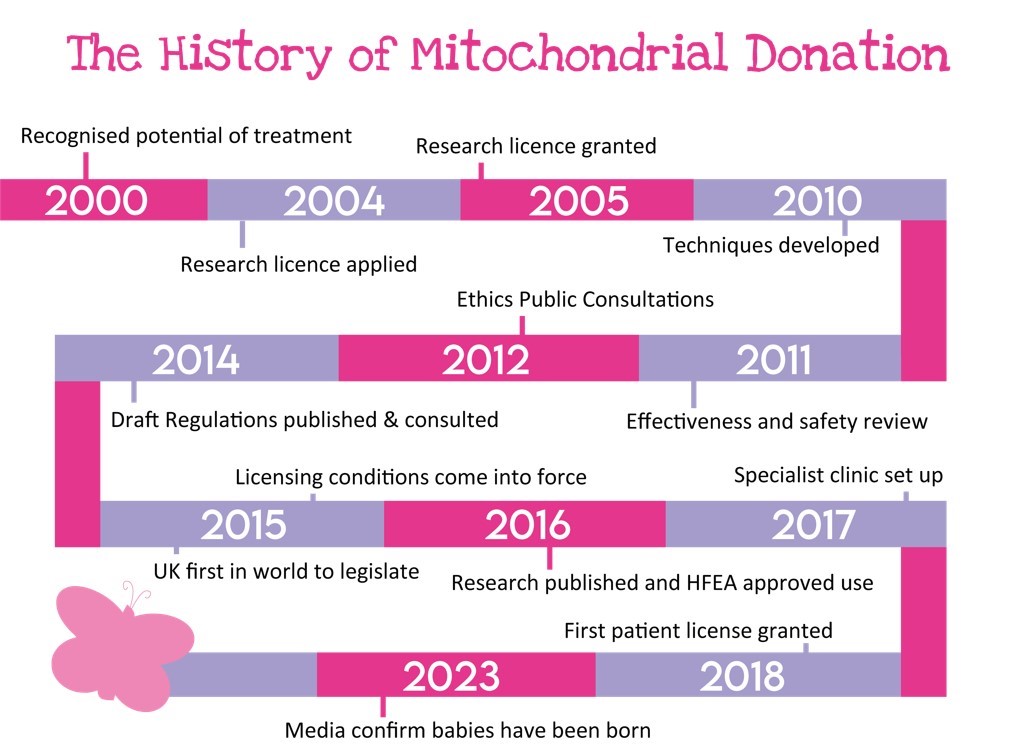
The story starts back in 2000...
- The Chief Medical Officer’s Expert Group Report, Stem Cell Research: Medical Progress with Responsibility considers and recognises the future potential use of the technique in treatment.
- May 2004 Newcastle University apply for a research licence to use PNT to avoid transmission of mitochondrial disease.
- September 2005 The HFEA research licence is granted on appeal.
- 2008 The Human Fertilisation and Embryology Act is passed, allowing researchers to develop techniques to prevent transmission of maternally inherited mitochondrial disease.
- 2010 Researchers at Newcastle University develop techniques to prevent diseased mitochondria being passed from mother to child.
- February 2011 The Human Fertilisation and Embryology Authority (HFEA) convene three Independent Expert Scientific Review panels to assess the effectiveness and safety of mitochondrial donation. The expert panel meets three times between 2011 and 2013. All their reports deem the techniques both safe and effective.
- June 2012 Nuffield Council on Bioethics publish “Novel techniques for the prevention of mitochondrial DNA disorders: an ethical review” and deem it ethical.
- September-December 2012 HFEA hold two Public Consultations.
- March 2013 Reports from these show general support for permitting mitochondrial donation, providing it is safely regulated, and ethical concerns are outweighed by benefits of preventing disease.
- February-May 2014 The Draft Regulations are published for public consultation.
- 3rd February 2015 The regulations are debated in the House of Commons and MPs given a free vote. Overwhelming cross-party support results in a vote of 382 Ayes and 128 Noes.
- 24th February 2015 Members of the Lords discuss the details of the regulations. A motion attempting to block their approval goes to a vote with 48 for and 280 against, so the draft regulations are agreed.
- 4th March 2015 Jane Ellison signs the Mitochondrial Donation Regulations with the UK being the first to legislate.
- 29th October 2015 The HEFA publish the licencing conditions for mitochondrial donation and these regulations come into force. The HFEA is now authorised to license and regulate mitochondrial donation.
Mitochondrial donation in the UK is now a reality. It can be taken out of the laboratory and offered to families that can benefit. Specialist centres will be allowed to apply to the HFEA for licenses on a case-by-case basis to offer this technique to severely affected families as a reproductive choice.
- 9th June 2016 The results of a study using the ‘pronuclear transfer’ technique in human embryos to prevent the transmission of mitochondrial DNA disorders are published in the journal Nature. The study was carried out by researchers at the Wellcome Centre for Mitochondrial Disease at Newcastle University and involved over 500 eggs from 64 donor women. Results of the study suggest that the technique would lead to normal pregnancies whilst also reducing the risk of babies having mitochondrial disease. The results of the study are to be considered by the Human Fertilisation and Embryology Authority (HFEA), who are to decide whether to issue the first licence to a clinic for the technique to be used in treatment.
- 30th November 2016 The HFEA expert scientific panel agree that there is sufficient evidence on mitochondrial donation to take the next step and proceed cautiously with offering this to families at high risk of passing mitochondrial disease onto their child. This is subject to final HFEA approval in December 2016.
- 15th December 2016 The HFEA give their final approval to mitochondrial donation. Clinics can now apply for licences on a case-by-case basis to treat families at high risk of passing on mitochondrial disease. NHS England will fund the first treatment trial for women who meet the HFEA criteria, so long as they agree to long-term follow up of children after they are born.
- 16th March 2017 Newcastle-upon-Tyne NHS Foundation Trust and Newcastle University are the first sites worldwide to be approved by the HFEA as authorised sites to undertake mitochondrial donation.
- March 2017 The first treatment application for mitochondrial donation is submitted to the HFEA for approval.
- April 2017 A new ‘reproductive choices service’ is set up in Newcastle to look after families with mitochondrial disease wishing to explore mitochondrial donation and other reproductive options.
- February 2018 the HFEA granted the first patient licence for mitochondrial donation to the Wellcome Centre for Mitochondrial Research at Newcastle University. The UK becomes the first country to legally offer the procedure to a patient.
- May 2023 The media confirms that at least one baby has since been born via the technique of mitochondrial donation.