What is Leigh Syndrome?
Leigh Syndrome, also known as Leigh’s disease, is a severe neurological disorder that typically emerges in infancy or early childhood. It’s caused by defects in the mitochondria, thus falls under the umbrella term of a mitochondrial disease.
Without enough energy, critical tissues, especially in the brain, begin to break down, leading to symptoms like muscle weakness, developmental delays and difficulty breathing.
Sadly, Leigh Syndrome is progressive and currently has no cure.
How are researchers tackling Leigh Syndrome?
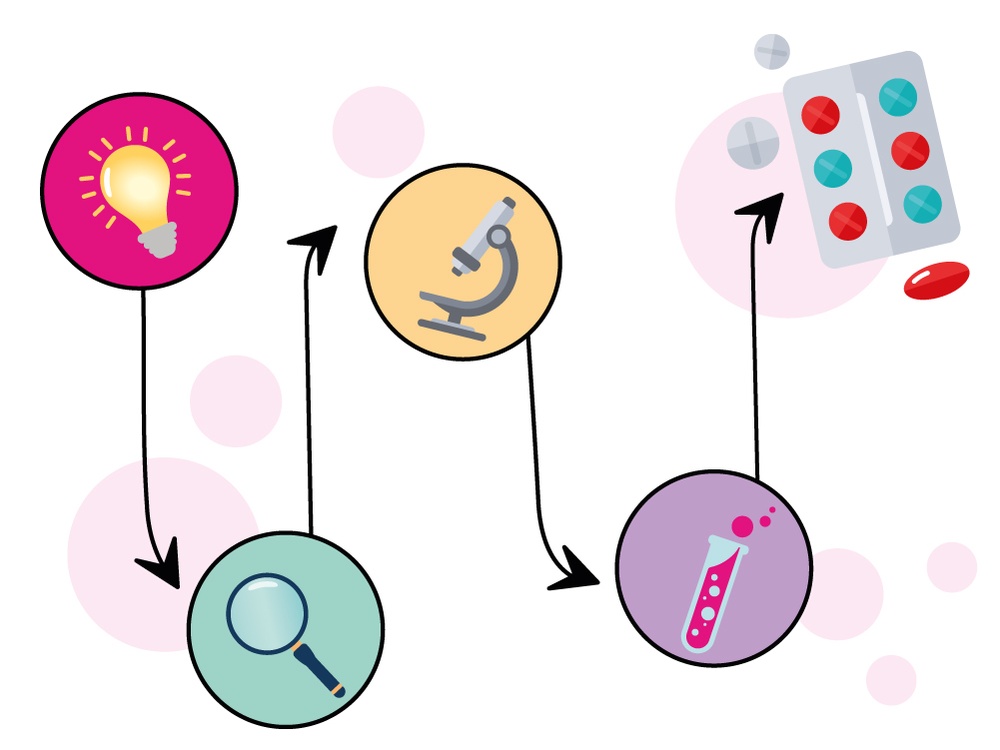
To better understand Leigh Syndrome and develop treatments, scientists use a variety of disease models in the lab.
A disease model is an experimental system, such as lab-grown cells, animals or organ-like structures, that mimics a human disease. Scientists use these models to study how a disease works and test potential treatments before trying them in humans.
For mitochondrial disease, models help researchers study how problems with mitochondria cause disease and test potential treatments. Each model provides a unique piece of the puzzle, giving scientists insights into the complex ways that defective mitochondria affect the body.
Different models, different insights
In a recently published paper, scientists explained the models currently being used to understand Leigh’s disease.
There are many different genetic errors that may cause Leigh Syndrome, which means no single model can capture all aspects of the disease. That’s why scientists use multiple research models, each offering a unique perspective:
- Yeast models: Yeast might seem an odd choice, but the cells that make up yeast share fundamental mitochondrial functions with human cells. By studying yeast, researchers can investigate the basic biochemistry of defective mitochondrial genes and how they impact energy production.
- Mouse models: Mice share a similar nervous system structure to humans so they can help scientists understand how mitochondrial mutations lead to neurological problems.
- Patient-derived stem cells: Scientists can take skin or blood cells from patients and turn them into stem cells. These are cells that can be turned into any cell in the body. This means that they can then be transformed into heart or brain cells. These lab-grown cells mimic real human tissues, allowing researchers to see how specific genetic mutations impact different organs.
- Organoids: Scientists can use stem cells to create mini-organs, or ‘organoids’, that behave like tiny versions of the brain or heart. These models offer an incredibly realistic way to test new treatments in a system that closely resembles a patient’s body. Whilst organoids represent an exciting development in the field, early work is still ongoing to understand how effectively they mimic human disease.
Why this research matters
These models are more than just tools for understanding the disease. They’re helping scientists find treatments for Leigh Syndrome.
Researchers can use them to test thousands of drugs, looking for chemicals that might boost energy production, protect cells from damage or even correct mitochondrial defects. Thanks to these breakthroughs, experimental treatments are being developed that might one day slow or even stop the progression of Leigh Syndrome.
Why there’s still more work to do
Whilst these models represent exciting progress in the field, with each helping to provide exciting insights into how we begin to understand and treat disease, there are many challenges that remain.
Leigh’s disease is caused by many different errors in a patient’s DNA and what works for one patient may not work for another. Additionally, treatments that show promise in the lab must go through rigorous clinical trials before they can be approved for patients. This takes a lot of time, funding and collaboration between researchers, doctors and families.
The future’s bright
While there is still a long road ahead, every discovery brings us closer to a cure and the more scientists develop these models, the more they can be used in other mitochondrial diseases. The advances made in research models are not just helping scientists understand Leigh Syndrome, they’re paving the way for real treatments. The journey is challenging, but with each breakthrough we move one step closer to a future where children diagnosed with Leigh’s disease have hope for a healthier life.
Behind the scenes at Lily, we’re actively working in the Leigh Syndrome space. We’re proud to be consortium partners in the Leigh Syndrome Roadmap Project (LSRP), a global initiative aimed at accelerating research and bringing potential treatments to patients faster. Stay tuned for exciting updates about the LSRP as we continue to discover what’s possible in Leigh Syndrome research.
You can read more about this Leigh Syndrome research at https://onlinelibrary.wiley.com/doi/10.1002/jimd.12804.